Rate of Reaction Calculation
View Rate-of-Reaction-calculation_2pdf from CHE MISC at University of the Cordilleras formerly Baguio Colleges Foundation. The mean rate of reaction can be calculated using either of these two equations.

Introduction To Reaction Rates Video Khan Academy
R k T A n B n.

. The rate of a chemical reaction is calculated in terms of a decrease in the concentration of any of the reactants per unit time or an increase in the concentration of any of the products per unit time. These equations will help you to calculate the mean rate of reaction. Download Rate of Reaction Cheat Sheet by clicking on the button below.
The rate of reaction is shown to be dependent on the concentration terms of reactant A and reactant B. View Rate of Reaction calculationpdf from BSN 101 at Saint Louis University Baguio City Main Campus - Bonifacio St Baguio City. The rate of the reaction is.
Rate of Reaction Rate of Reaction. Rate of disappearance of R Decrease in concentration of Rtime taken -ΔRΔt. No matter which quantity is measured during the course of a reaction the average rate of reaction can be calculated using the equation below.
Rate of reaction has units. And rate of appearance of P Increase in. For example the rate of reaction of C is.
First the general rate of reaction formula that involves the rate constant for a general reaction equation. Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. A A b B p P q Q.
I recommend watching this in x125 - 15 speed In this video we start the series of videos on reaction rates by going over rate of formation and rate of con. K T is the rate constant or reaction rate coefficient. This expression is termed Rate law.
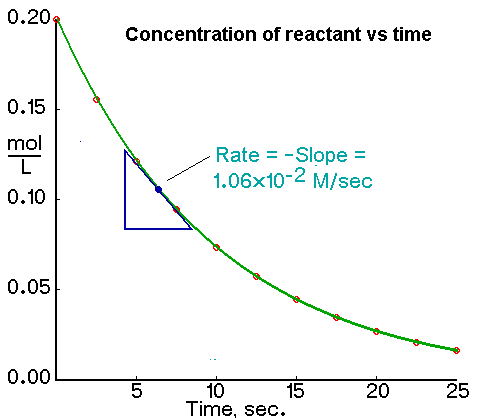
The rate of a chemical equation can be calculated using the rate equation. From Figure the gradient of the tangent at any point on the curve This is the rate of reaction at that given time. Or Rate K A α B β.
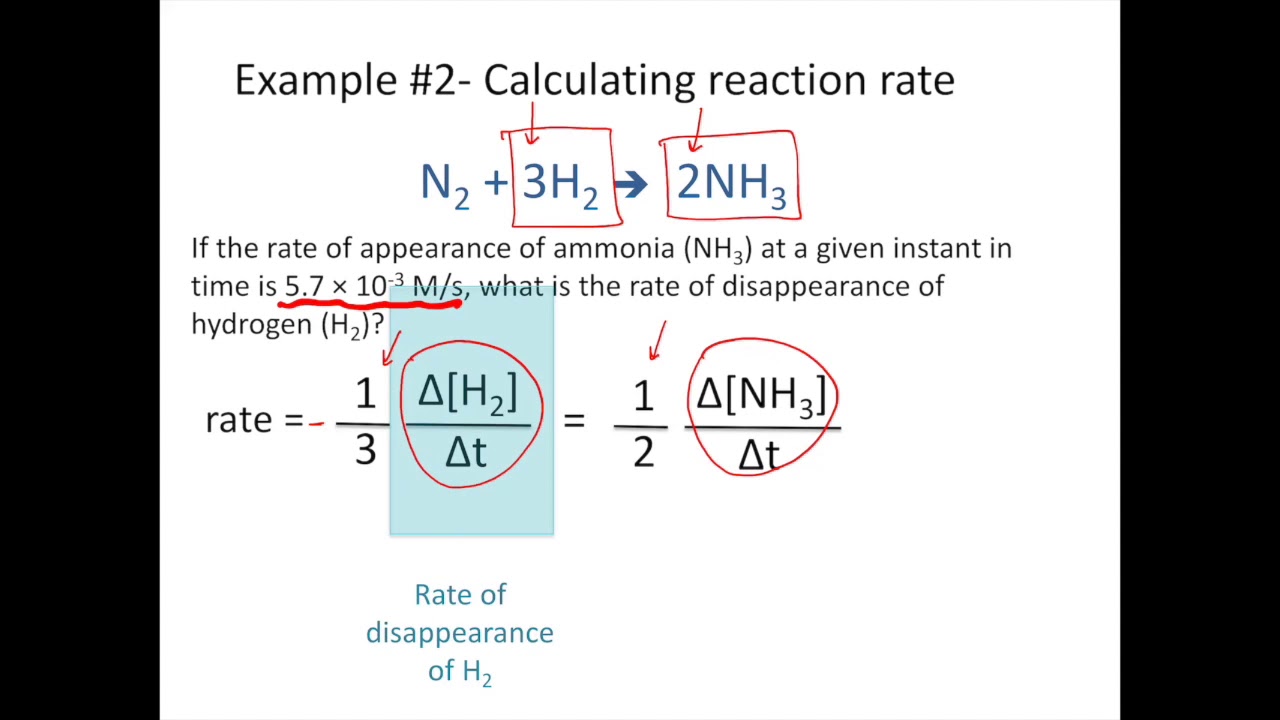
In this equation K stands for Rate constant. If 30g of Silver Chloride was produced in reaction within 15 seconds. So we divide the rate of each component by its coefficient in the chemical equation.
Hence Rate of reaction at a given time gradient of the curve at that instant The rate of reaction at a given time t can be calculated through the following steps. How do you calculate the rate of a chemical reaction. Rate of Reaction Rate of Reaction Measure the changes in.
If the product or reactant is a solid then they will be measured in grams. EqaA bB rightleftharpoons cC dD rate kAnBo eq Where n and o are. There are two simple equations we need to know for exams.
For a chemical reaction. Then Rate of reaction A α B β. GCSE Chemistry Calculating Rates of Reaction Units.
Rate of reaction of C ΔCΔt The overall rate of reaction should be the same whichever component we measure. Calculate the mean rate of reaction from 0 to 15 seconds. Rate of reaction amount of products formed.
Units of rate constant are. The rate of reaction is the amount of products formed per unit time. Determining the rate of reaction at a given time from the graph.
Rate of a reaction amount of products formedtime. Calculate the rate of reaction. These are gs or cm 3 s.
If the product or. I Unit L-1 mol s-1 Zero order reaction. The rate of reaction is measured in two units.
Rate of a reaction amount of reactants usedtime. Equation of Rate of a Chemical Reaction. Rate of reaction 1aΔAΔt 1bΔBΔt 1cΔCΔt 1dΔDΔt EXAMPLE.
18 Mean rate of reaction quantity of product formed time taken Mean rate of reaction 30 15 Mean rate of reaction 2 cm3s.

How Do You Calculate The Reaction Rate A Plus Topper

No comments for "Rate of Reaction Calculation"
Post a Comment